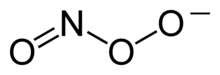
Peroxynitrit
Peroxynitrit je anion se vzorcem ONOO−. Je to nestabilní valenční izomer aniontu NO3−, který má stejný sumární vzorec, ale odlišnou strukturu. Ačkoliv je kyselina peroxynitridová vysoce reaktivní, její konjugovaná báze peroxynitrit je stabilní.[1] Vyrábí se reakcí peroxidu vodíku s dusitanem:
- H2O2 + NO2− → ONOO− + H2O
Peroxynitrit je oxidační a nitrační činidlo. Díky svým oxidačním vlastnostem může napadat široké spektrum biomolekul v buňkách, včetně DNA a bílkovin. Formování peroxynitritu in vivo je přičítáno reakci volných radikálů peroxidu s volnými radikály oxidu dusnatého.[2]:
- ·O2− + ·NO → ONO2−
Produktem reakce těchto dvou radikálů je peroxinitrit, molekula, která sama o sobě není radikálem, ale má silné oxidační vlastnosti.
V laboratoři může být roztok peroxynitritu připraven reakcí okyseleného peroxidu vodíku s roztokem dusitanu sodného, a následovaným rychlým přidáním hydroxidu sodného. Koncentrace je indikována absorbancí při 302 nm (pH 12, λ302 = 1670 M−1 cm−1).[3]
Peroxynitrit (spíše než NO·) je in vivo odpovědný za nitraci a hydroxylaci aminokyseliny tyrozinu. Přechodné kovy včetně kovů v aktivních centrech superoxiddizmutázy a myeloperoxidázy katalyzují jeho heterolytické štěpení na hydroxidový anion a nitroniový kation, jež je schopen napadat fenolické sloučeniny (in vivo například tyrozin na 3-nitrotyrozin).
Reference
V tomto článku byl použit překlad textu z článku Peroxynitrite na anglické Wikipedii.
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ Pacher, P.; Beckman, J. S.; Liaudet, L.; “Nitric Oxide and Peroxynitrite: in Health and disease” Physiological Reviews 2007, volume 87(1), page 315-424. PMID 17237348
- ↑ Beckman, J. S.; Koppenol, W. H. “Nitric Oxide, Superoxide, and Peroxynitrite: the Good, the Bad, and Ugly” American Journal of Physiology- Cell Physiology 1996, volume 271, page C1424-C1437.
Externí odkazy
 Obrázky, zvuky či videa k tématu Peroxynitrit na Wikimedia Commons
Obrázky, zvuky či videa k tématu Peroxynitrit na Wikimedia Commons
Média použitá na této stránce
Autor: Erica Novo and Maurizio Parola, Licence: CC BY 2.0
Reactions of peroxynitrite leading to either apoptotic or necrotic cell death. NO and RNS may potentially prevent hepatocyte apoptosis as well as promote either necrotic or apoptotic cell death. The following mechanisms have been proposed. With regard to NO, RNS and prevention of apoptosis, the main molecular mechanisms resulting in an anti-apoptotic effect, related to S-nitrosating species, include [237-239]: stimulation of guanylate cyclase, leading to increased cyclic guanine monophosphate levels; the evolutionarily conserved inhibition of caspases by potentially reversible S-nitrosation of a critical cysteine residue at the caspase active site; activation of the Ras/Erk1/2 pro-survival pathway, which may result in activation of mitogen and stress activated kinase 1 (MSK1) and pp90 ribosomal S6 kinase (RSK), which in turn may inactivate the pro-apoptotic protein Bad or up-regulate anti-apoptotic proteins of the Bcl-2 family [237]; RNS also possibly acting by inhibiting leukocyte adhesion through S-nitrosation of critical -SH groups exposed by activated neutrophils and macrophages [240]. NO and RNS may prevent or promote cell death in relation to intracellular and intramitochondrial (because of mitochondrial NOS) levels of GSH and the concomitant cellular levels of transition metal ions. Moreover, NO may also lead to up-regulation of heme oxygenase 1 (HO-1) in hepatocytes and this may serve as a cytoprotective event [237,238]. The dark (that is, damaging) side of NO and RNS: in the presence of higher levels of ROS, the right NO/superoxide ratio or levels of molecular oxygen, NO may lead again to generation of highly reactive RNS, such as N2O3 or ONOO- at levels that are able to induce more aggressive oxidation, nitrosation/S-nitrosation and nitration of different biological macromolecules, potentially leading either to necrotic or apoptotic cell death. If NO-dependent pro-apoptotic mechanisms are concerned, the following have been shown to have a major role, with some again depending on S-nitrosating species: RNS and so called NO+ -carriers (nitrosating species) may result in activation of JNK, which, as previously reported for ROS, may sustain induction of apoptosis; NO, if generated at high levels in mitochondria, may result in ubiquinol auto-oxidation with concomitant production of superoxide, hydrogen peroxide and ONOO-, species that may be responsible for irreversible damage to complexes I and II of the respiratory chain, inhibition of ATP synthesis and eventually cytochrome c release and induction of caspase-dependent apoptosis. It should also be noted that, in the presence of significant redox stress, NO can potentiate damaging effects, resulting in a scenario of necrotic cell death rather than apoptosis. This is likely to occur particularly when the redox state is significantly affected, as in conditions resulting in depletion of GSH or significant alterations of the GSH/GSSG ratio. Novo and Parola Fibrogenesis & Tissue Repair 2008 1:5 doi:10.1186/1755-1536-1-5